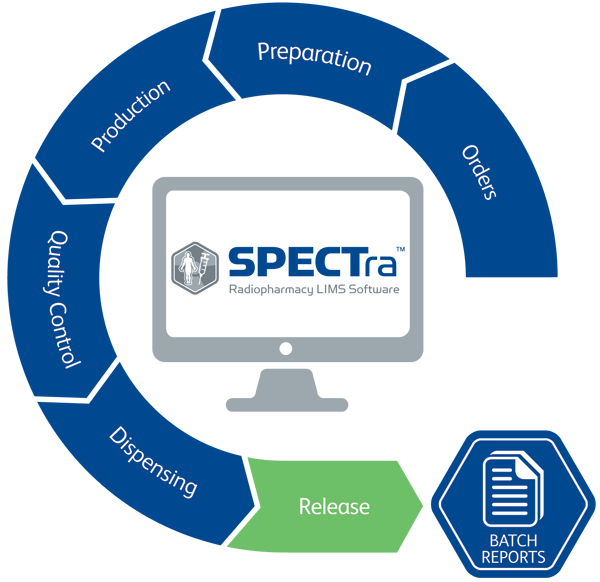
A purpose built Radiopharmacy Laboratory Information Management System
SPECTra is a true Radiopharmacy Laboratory Information Management System (LIMS), designed specifically to improve efficiency and compliance.
SPECTra is based on our market leading PET LIMS system, PETra. Since its introduction to the market PETra has quickly become the industry standard following successful installations in some of the world’s most prestigious and regulated PET facilities.
Direct Data Capture
During the various phases of the workflow, facilities have to manage data from a range of equipment and software packages, resulting in multiple outputs and reports. What’s unique about SPECTra Radiopharmacy LIMS, is that it captures data directly from all equipment used in the process.
Eliminates Transcription Errors
As a result of direct data capture, SPECTra Radiopharmacy LIMS completely eliminates manual transcription and the likelihood of any errors.
Interfaces to all Equipment
The state of the art radiopharmacy information management system captures data from all the equipment either directly or by interfacing to the respective software.
Improved Efficiency in your Radiopharmacy
By managing data electronically SPECTra significantly improves workflow efficiency in the following areas:
- Customer orders with consolidated invoicing information.
- Pre-production checks and worksheets.
- Direct capture from equipment, thus eliminating transcription errors.
- Dose Requirements storing site, customer and dose information in one manageable place.
- Barcode driven inventory management, providing you with up to date stock levels and allowing use of accepted raw material only.
- Quality Management System; SOP, CAPA, Deviation, Change Control, OOS and Trending.
- Instrument Maintenance / Calibration; ensuring equipment is maintained in accordance with your SOPs.
- Labels and shipping documents.
- Notifications for efficient communications and reminders of any tasks.
- Training / User Records.
- Audit Trail.
- Sub-batches / Drug Stability Testing.
- Security Access in accordance with regulatory requirements.
- Electronic signatures; no more missing manual signatures.
Improved Radiopharmacy Compliance
SPECTra is a secure Radiopharmacy LIMS that ensures compliance with regulatory demands.
User access is managed via a unique login ID that is linked to users’ training and skill set. Electronic signatures and audit trails are configurable and in line with the FDA 21 CFR part 11 requirements in sections 11.50 and 11.10, respectively. These functionalities ensure that you don’t miss signatures where required and that retrieving audit trails for review is the effort of a few mouse clicks.
LabLogic have decades of experience creating systems within highly regulated environments. We are confident that our systems will improve compliance within your facility.
Standardize Radiopharmacy Production
Multi-site facilities can standardize production of their radiopharmaceuticals with SPECTra. Each site can be configured to ensure the same processes are being routinely carried out, removing inconsistencies.
Implementing SPECTra enables LabLogic experts, alongside end user QA, to review and challenge current practices, building improvements and industry best practice into existing processes.
Request a demonstration today, or get in touch with us to talk about how your SPECTra radiopharmacy LIMS requirements.